Click on the infographic below for a full overview of the ADAURA trial and updated 5-year data
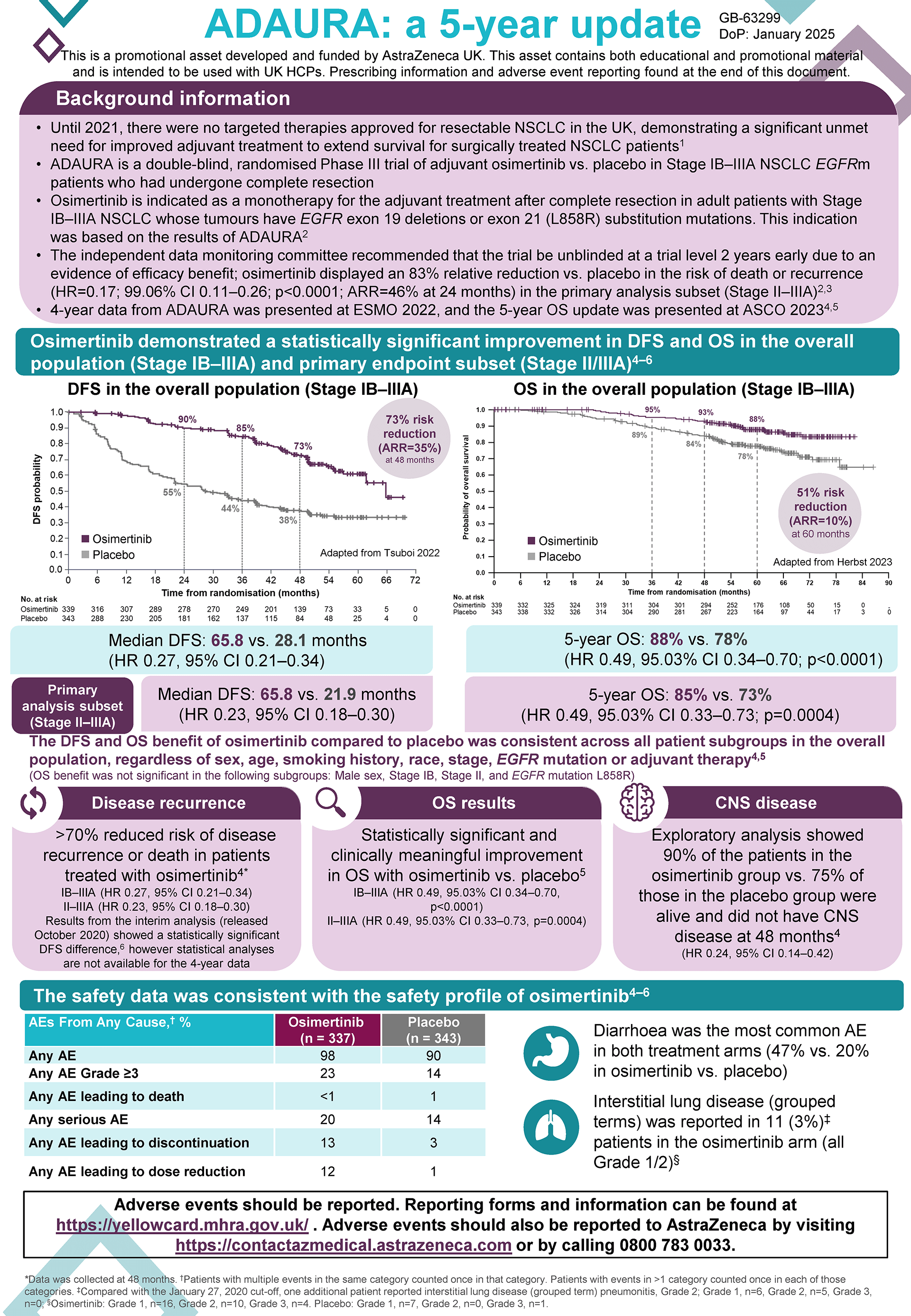
Background
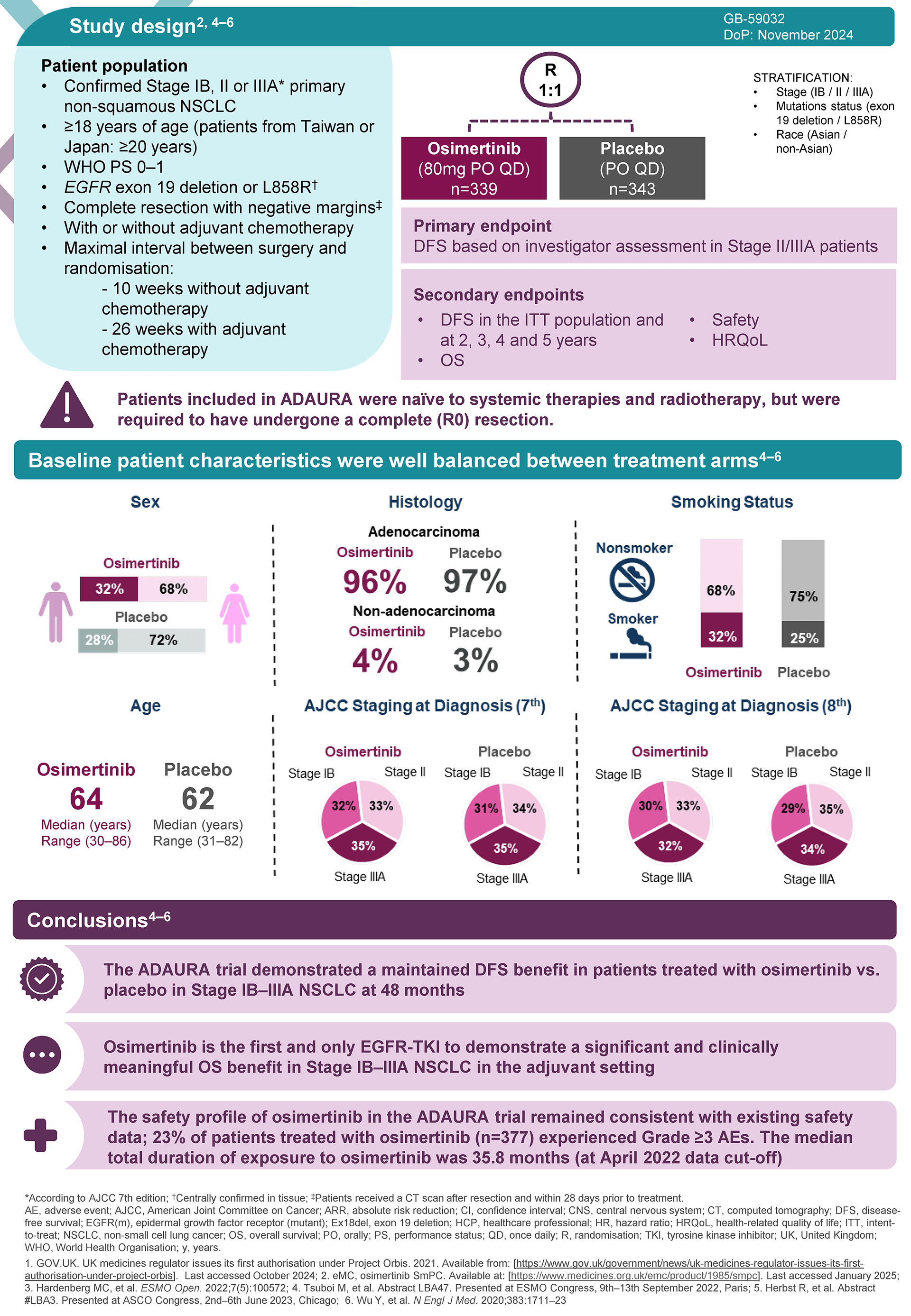
The ADAURA trial was a double-blind, phase 3 trial which randomly assigned patients with completely resected EGFRm NSCLC to receive either Tagrisso (osimertinib) or placebo for 3 years.
Conclusion
Adjuvant TAGRISSO demonstrated a statistically significant and clinically meaningful improvement in OS vs. placebo in the recent 5-year readout of ADAURA, reiterating its long-term efficacy. In patients with stage II-IIIA disease (primary endpoint subset), the relative reduction in risk of death was 51%, HR: 0.49, 95.03% CI 0.33-0.73; P=0.0004, ARR: 12%.
Further information on ADAURA Trial
ADAURA Trial: 4 year DFS update
Dr Yvonne Summers, Consultant Medical Oncologist, Christie NHS Foundation Trust
Please click here for TAGRISSO UK prescribing information
ADAURA Trial: CNS recurrence data
Dr John Conibear, Consultant Clinical Oncologist, Barts Health NHS Trust
Please click here for TAGRISSO UK prescribing information
ADAURA Trial: Evolving UK clinical practice for EGFRm+ NSCLC
Dr Yvonne Summers, Consultant Medical Oncologist, Christie NHS Foundation Trust
Dr Shobhit Baijal, Consultant Medical Oncologist, Birmingham University Hospitals NHS Foundation Trust
Please click here for TAGRISSO UK prescribing information
References
- Tsuboi M, et al. Abstract LBA47. Presented at ESMO Congress, 9th–13th September 2022, Paris