GB-59033
Date of Preparation: January 2025
The content on this website is intended for UK Healthcare professionals only. The website contains promotional content.
Swipe across to see full table
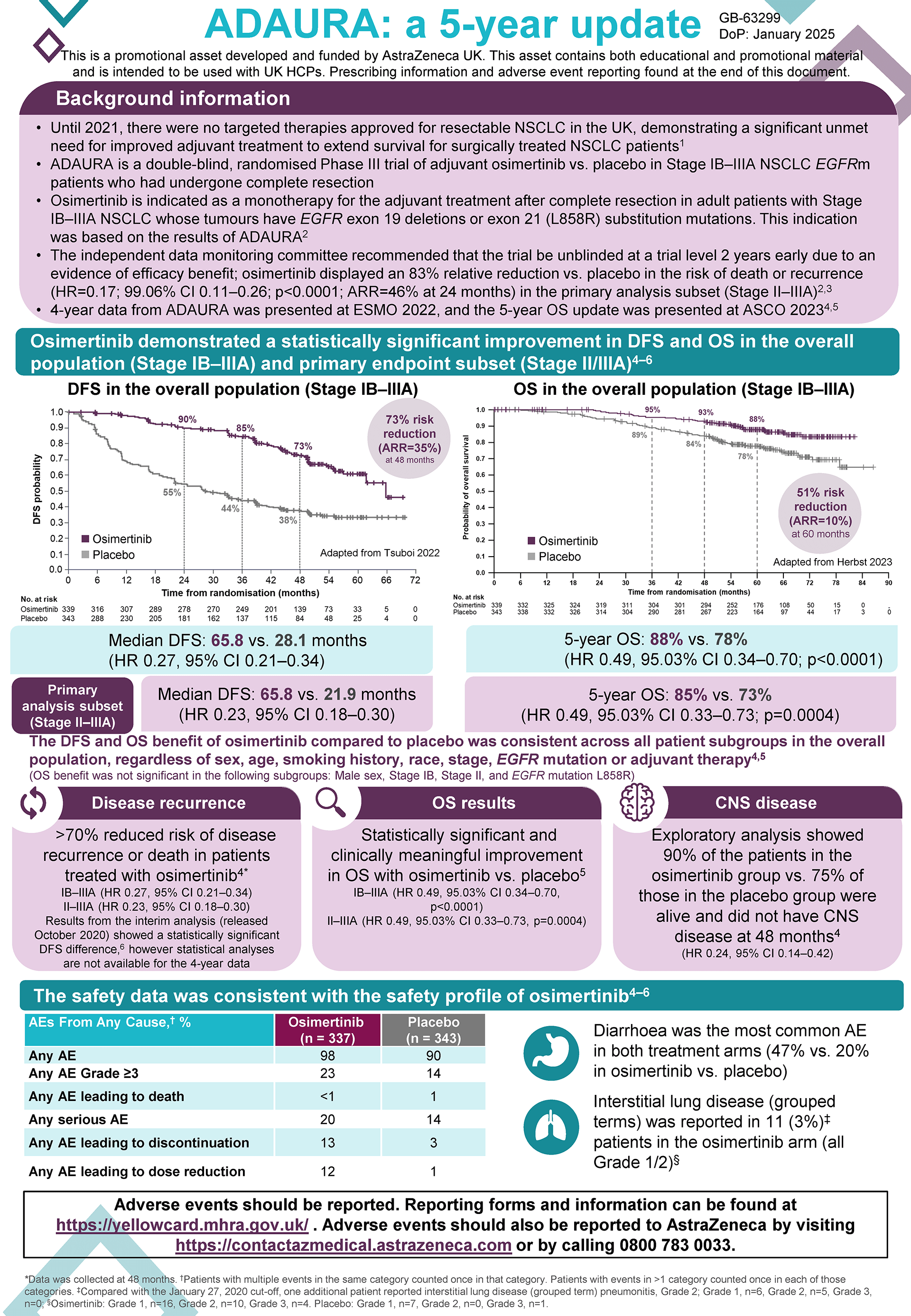
| Adverse reactions reported | TAGRISSO | TAGRISSO with pemetrexed and platinum-based chemotherapy | ||
|---|---|---|---|---|
| CIOMS descriptor/ overall frequency (all CTCAE grades) | Frequency of CTCAE grade 3 or higher | CIOMS descriptor/ overall frequency (all CTCAE grades) | Frequency of CTCAE grade 3 or higher | |
| Blood and lymphatic system disorders | ||||
| Aplastic anaemia | Rare (0.06%) | 0.06% | 0% | 0% |
| Leukopenia | Common (5.4%) | 0.4% | Very common (12.7%) | 2.9% |
| Lymphopenia | Common (1.7%) | 0.2% | Common (2.5%) | 1.1% |
| Thrombocytopenia | Common (7.6%) | 0.6% | Very common (18.5%) | 6.9% |
| Neutropenia | Common (6%) | 0.9% | Very common (24.6%) | 13.4% |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | Very common (19%) | 1.2% | Very common (31%) | 2.9% |
| Eye disorders | ||||
| Keratitis | Uncommon (0.6%) | 0.06% | Uncommon (0.7%) | 0% |
| Cardiac disorders | ||||
| Cardiac failure | Uncommon (0.5%) | 0.2% | Common (1.8%) | 1.1% |
| Respiratory, thoracic and mediastinal disorders | ||||
| Epistaxis | Common (6%) | 0% | Common (7%) | 0.4% |
| Interstitial lung disease | Common (4.0%) | 1.4% | Common (3.3%) | 0.7% |
| Gastrointestinal disorders | ||||
| Diarrhoea | Very common (47%) | 1.4% | Very common (43%) | 2.9% |
| Stomatitis | Very common (24%) | 0.4% | Very common (31%) | 0.4% |
| Skin and subcutaneous tissue disorders | ||||
| Rash | Very common (46%) | 0.8% | Very common (49%) | 2.5% |
| Paronychia | Very common (34%) | 0.4% | Very common (27%) | 0.7% |
| Dry skin | Very common (32%) | 0.1% | Very common (24%) | 0% |
| Pruritus | Very common (17%) | 0.06% | Common (8%) | 0% |
| Alopecia | Common (5%) | 0% | Common (9%) | 0% |
| Palmar-plantar erythrodysaesthesia syndrome | Common (2.1%) | 0% | Common (5%) | 0% |
| Urticaria | Common (1.9%) | 0.1% | Common (1.4%) | 0.4% |
| Skin hyperpigmentation | Common (1.0%) | 0% | Common (2.5%) | 0% |
| Erythema multiforme | Uncommon (0.3%) | 0% | Common (1.4%) | 0.7% |
| Cutaneous vasculitis | Uncommon (0.2%) | 0% | 0% | |
| Stevens-Johnson syndrome | Rare (0.02%) | 0% | 0% | |
| Toxic Epidermal Necrolysis | Not known | 0% | 0% | |
| Investigations | ||||
| Left ventricular ejection fraction decreased | Common (4.2%) | Common (8%) | ||
| QTc interval prolongation | Common (1.1%) | Common (1.8%) | ||
| Blood creatine phosphokinase increased | Common (1.9%) | 0.3% | Common (3.3%) | 1.1% |
| Investigations (Findings based on test results presented as CTCAE grade shifts) | ||||
| Leucocytes decreased | Very common (65%) | 1.8% | Very common (88%) | 20% |
| Lymphocytes decreased | Very common (64%) | 8% | Very common (78%) | 16% |
| Platelet count decreased | Very common (53%) | 1.3% | Very common (85%) | 16% |
| Neutrophils decreased | Very common (36%) | 4.0% | Very common (85%) | 36% |
| Blood creatinine increased | Common (9%) | 0.2% | Very common (22%) | 0.4% |
| Musculoskeletal and connective tissue disorders | ||||
| Myositis | Uncommon (0.2%) | 0% | 0% | 0% |
Consult SmPC for further information and detail, including adverse events and management. Note: ILD, severe cutaneous adverse reactions, QTc interval prolongation, keratitis, and aplastic anaemia are special warnings for Tagrisso.
1. Tagrisso. Summary of Product Characteristics.
GB-64711 | March 2025