Tagrisso Case Studies
View cases by PROFILE (i.e. early stage, first line, second line) or by FORMAT
(i.e. podcasts, videos, interactive module) by selecting from the dropdown
View cases by PROFILE (i.e. early stage, first line, second line) or by FORMAT
(i.e. podcasts, videos, interactive module) by selecting from the dropdown
GB-57396
Date of Preparation: January 2025
The content on this website is intended for UK Healthcare professionals only. The website contains promotional content.
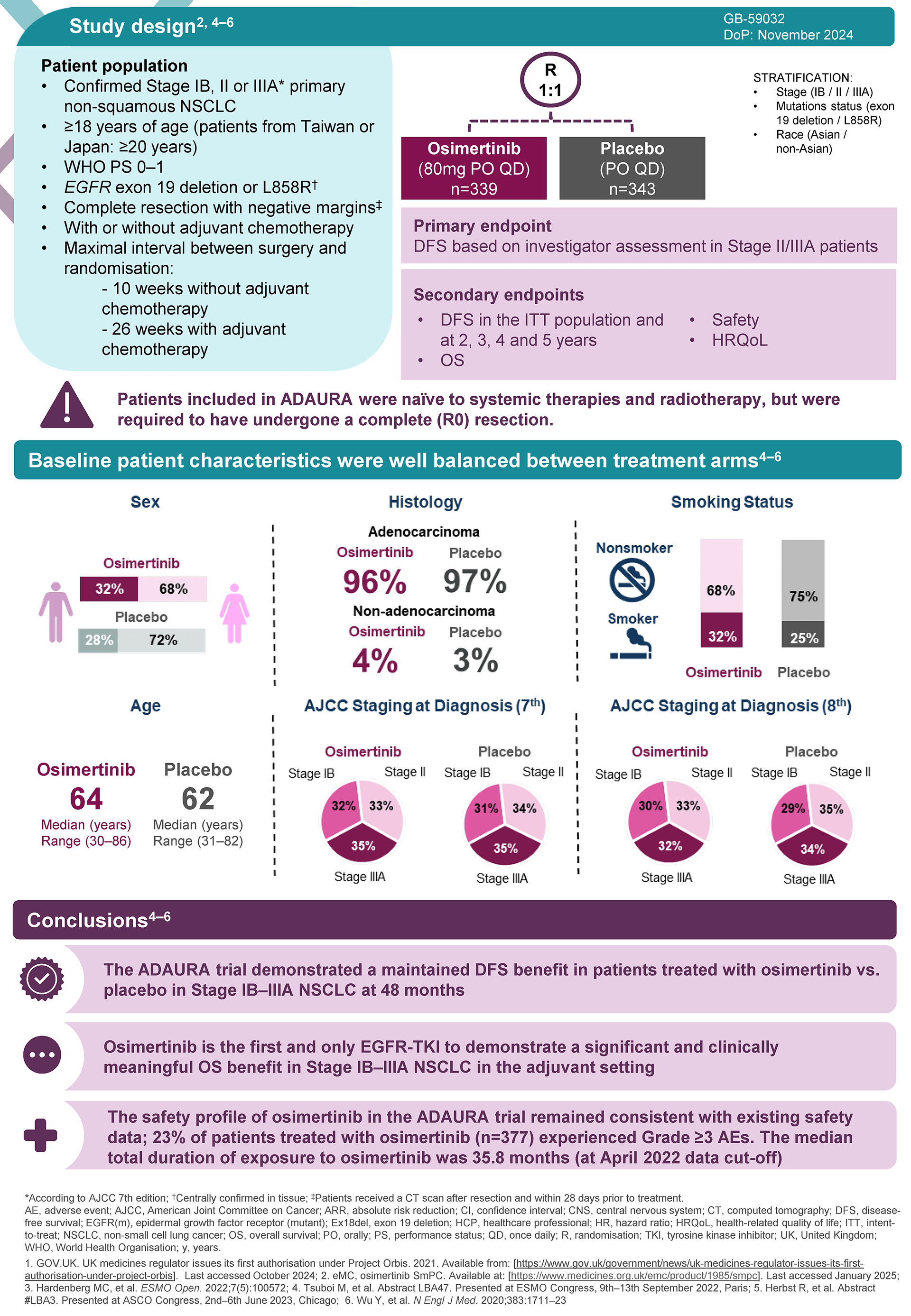
Swipe across to see full table
| Adverse reactions reported | TAGRISSO | TAGRISSO with pemetrexed and platinum-based chemotherapy | ||
|---|---|---|---|---|
| CIOMS descriptor/ overall frequency (all CTCAE grades) | Frequency of CTCAE grade 3 or higher | CIOMS descriptor/ overall frequency (all CTCAE grades) | Frequency of CTCAE grade 3 or higher | |
| Blood and lymphatic system disorders | ||||
| Aplastic anaemia | Rare (0.06%) | 0.06% | 0% | 0% |
| Leukopenia | Common (5.4%) | 0.4% | Very common (12.7%) | 2.9% |
| Lymphopenia | Common (1.7%) | 0.2% | Common (2.5%) | 1.1% |
| Thrombocytopenia | Common (7.6%) | 0.6% | Very common (18.5%) | 6.9% |
| Neutropenia | Common (6%) | 0.9% | Very common (24.6%) | 13.4% |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | Very common (19%) | 1.2% | Very common (31%) | 2.9% |
| Eye disorders | ||||
| Keratitis | Uncommon (0.6%) | 0.06% | Uncommon (0.7%) | 0% |
| Cardiac disorders | ||||
| Cardiac failure | Uncommon (0.5%) | 0.2% | Common (1.8%) | 1.1% |
| Respiratory, thoracic and mediastinal disorders | ||||
| Epistaxis | Common (6%) | 0% | Common (7%) | 0.4% |
| Interstitial lung disease | Common (4.0%) | 1.4% | Common (3.3%) | 0.7% |
| Gastrointestinal disorders | ||||
| Diarrhoea | Very common (47%) | 1.4% | Very common (43%) | 2.9% |
| Stomatitis | Very common (24%) | 0.4% | Very common (31%) | 0.4% |
| Skin and subcutaneous tissue disorders | ||||
| Rash | Very common (46%) | 0.8% | Very common (49%) | 2.5% |
| Paronychia | Very common (34%) | 0.4% | Very common (27%) | 0.7% |
| Dry skin | Very common (32%) | 0.1% | Very common (24%) | 0% |
| Pruritus | Very common (17%) | 0.06% | Common (8%) | 0% |
| Alopecia | Common (5%) | 0% | Common (9%) | 0% |
| Palmar-plantar erythrodysaesthesia syndrome | Common (2.1%) | 0% | Common (5%) | 0% |
| Urticaria | Common (1.9%) | 0.1% | Common (1.4%) | 0.4% |
| Skin hyperpigmentation | Common (1.0%) | 0% | Common (2.5%) | 0% |
| Erythema multiforme | Uncommon (0.3%) | 0% | Common (1.4%) | 0.7% |
| Cutaneous vasculitis | Uncommon (0.2%) | 0% | 0% | |
| Stevens-Johnson syndrome | Rare (0.02%) | 0% | 0% | |
| Toxic Epidermal Necrolysis | Not known | 0% | 0% | |
| Investigations | ||||
| Left ventricular ejection fraction decreased | Common (4.2%) | Common (8%) | ||
| QTc interval prolongation | Common (1.1%) | Common (1.8%) | ||
| Blood creatine phosphokinase increased | Common (1.9%) | 0.3% | Common (3.3%) | 1.1% |
| Investigations (Findings based on test results presented as CTCAE grade shifts) | ||||
| Leucocytes decreased | Very common (65%) | 1.8% | Very common (88%) | 20% |
| Lymphocytes decreased | Very common (64%) | 8% | Very common (78%) | 16% |
| Platelet count decreased | Very common (53%) | 1.3% | Very common (85%) | 16% |
| Neutrophils decreased | Very common (36%) | 4.0% | Very common (85%) | 36% |
| Blood creatinine increased | Common (9%) | 0.2% | Very common (22%) | 0.4% |
| Musculoskeletal and connective tissue disorders | ||||
| Myositis | Uncommon (0.2%) | 0% | 0% | 0% |
Consult SmPC for further information and detail, including adverse events and management. Note: ILD, severe cutaneous adverse reactions, QTc interval prolongation, keratitis, and aplastic anaemia are special warnings for Tagrisso.
1. Tagrisso. Summary of Product Characteristics.
GB-64711 | March 2025